Our question was: How are Molecular Bonds formed between atoms and in what way?
Preliminary Info: we had drawn many of Lewis' structures and read our book before going into this lab. We also knew how the structures of molecules formed based upon repelling groups and electron pairs.
Hypothesis: Well we hypothesized that this would be fun! :) (and educational)
Materials: 1 Model Kit for molecular bonding, including 5 carbon atoms, 15 regular bonding shafts, 5 bendy bonding shafts, 7 oxygen, 3 nitrogen, 8 hydrogen, 6 fluorine, also we had reference work sheets pertaining to the molecular geometry of molecules.
Safety: Just kidding, you just don't put them in your mouth. Do not throw objects in the chemistry room!!!!
 Procedure: we first opened the plastic bag containing our materials. we were assigned to do a worksheet containing multiple chemical equations such as H2O. We constructed all the chemicals in to 3D models. After completing the task a making the structure we were to draw a 2D diagram of the structure onto the worksheet. The worksheet also asked for a Lewis structure, the actual molecular geometry, the bond angle, and weather it is polar, or if it is a resonance structure.
Procedure: we first opened the plastic bag containing our materials. we were assigned to do a worksheet containing multiple chemical equations such as H2O. We constructed all the chemicals in to 3D models. After completing the task a making the structure we were to draw a 2D diagram of the structure onto the worksheet. The worksheet also asked for a Lewis structure, the actual molecular geometry, the bond angle, and weather it is polar, or if it is a resonance structure.Results: We completed our assignment with smiles and learned about the structural geometry of molecules. The once two dimensional ideas of what molecules should be have been remodeled in our thoughts as objects as tangible as ourselves.
1. Explain how water's shape causes it to be polar?
Waters shape is bent therefore one side has a greater polarity than the other.
2. Describe how waters properties would be different if the molecules were linear instead of bent?
Water would loss its polarity therefore it would no longer be able to dissolve substances
3. Based on the results of this experiment. List the molecules from the experiment that would soluble.
HF, IF3, SIH2O, CH3NH2, BF3
Following are the physical results of our lab. Enjoy.
C3H8


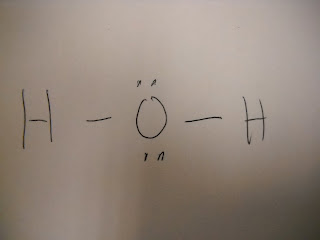

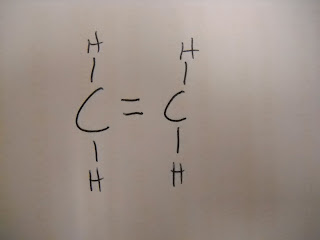
C2H4

H2O2


SF6


Si2H6


In conclusion: We had fun learning how to recognize, construct, and organize molecular structures.
